SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution
Identifying patterns of genomic scarring in ovarian cancer samples
Accurate identification and reporting of Homologous Recombination Deficiency (HRD) status are critical for better patient management.
SOPHiA DDM™ Dx HRD Solution is a CE-marked in vitro diagnostic (IVD) application leveraging low-pass Whole Genome Sequencing (WGS) and a proprietary deep-learning algorithm. Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application determines HRD status of tumors, helping healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient care.
Reduced turnaround time with a ready-to-sequence target-enriched library in just 1.5 days
In-house deep-learning algorithm revealing extent of genomic scarring through the Genomic Integrity Index (GII)
CE-IVD marked SOPHiA DDM™ Platform web for intuitive reporting with unlimited and safe data storage
Product Details
Efficiently reveal genomic scarring with a unique score
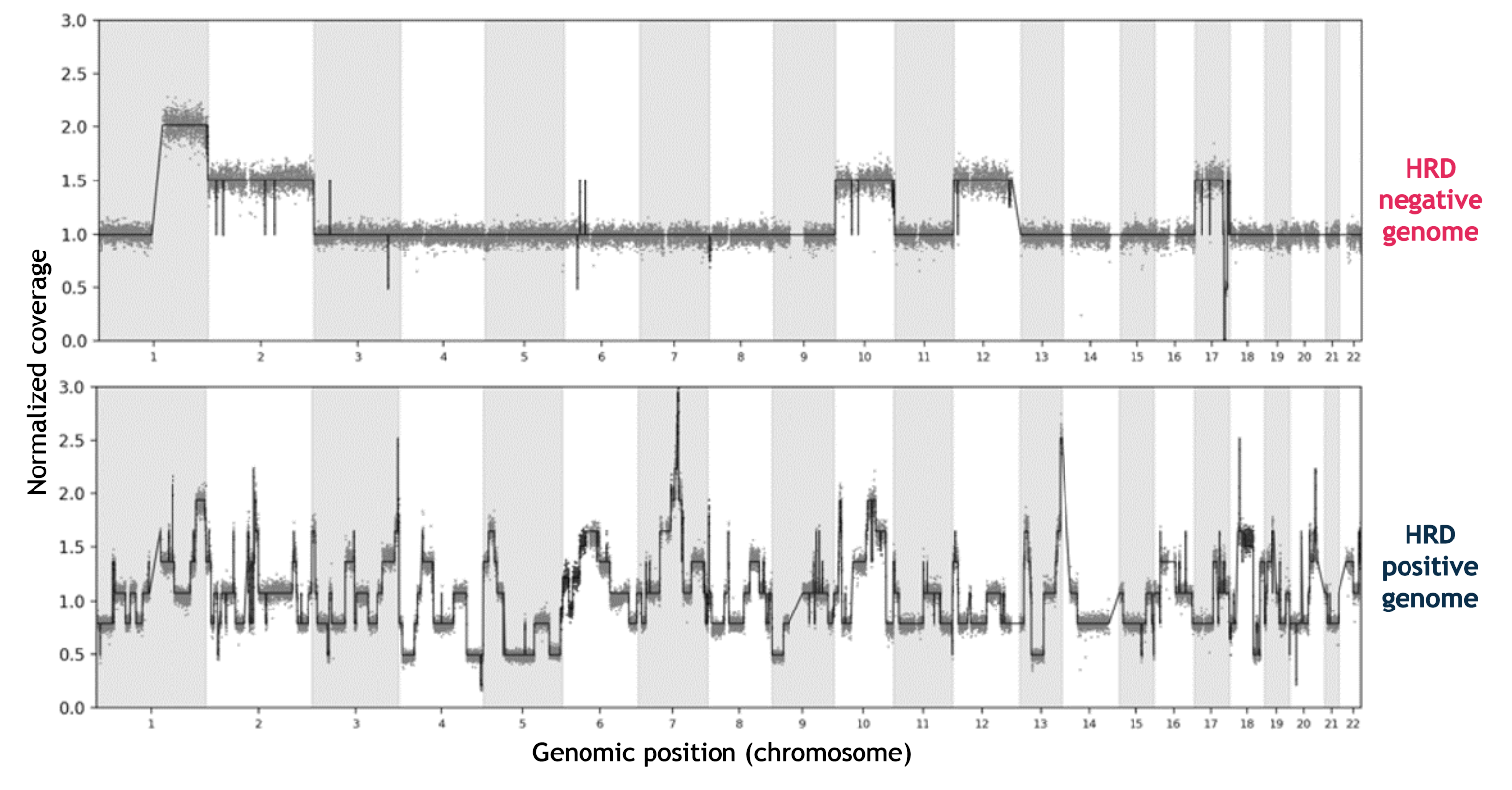
SOPHiA DDM™ Dx HRD Solution makes the most of low-pass WGS in conjunction with a convolutional neural network-based deep-learning algorithm to produce the Genomic Integrity Index (GII). This score measures the extent of genomic scarring across the genome as a result of mutations within genes associated with homologous recombination repair (HRR), yielding HRD impact on tumor samples.
Data on file
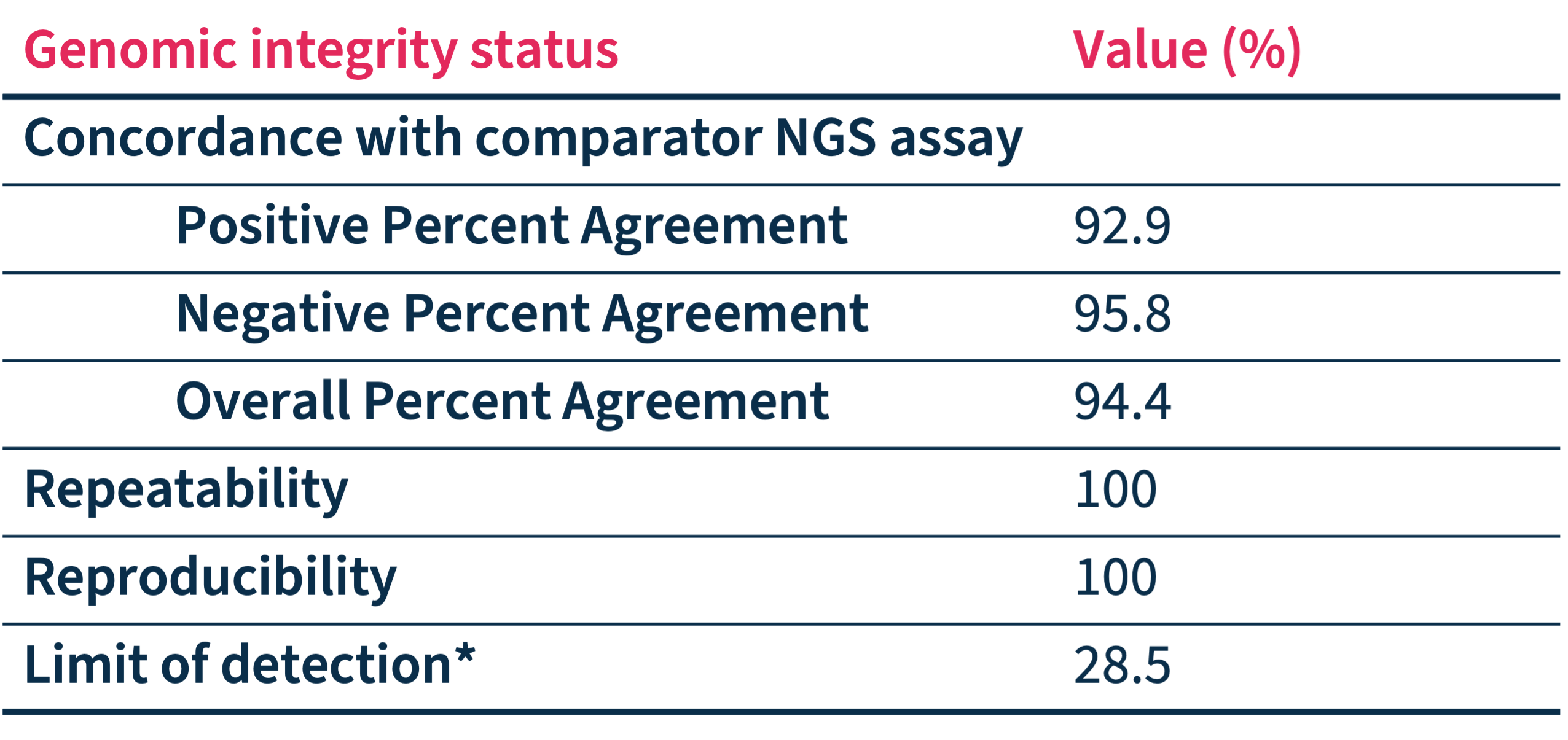
* Established by measuring the lowest tumor content for which sensitivity is larger or equal to 95%.
Accurately detect HRD
SOPHiA DDM™ Dx HRD Solution accuracy has been assessed through a multicenter performance evaluation study* showing:
- Reliable genomic integrity status determination
- Low rejection rate
- Clinical-grade analytical performance that can facilitate accelerated and confident decision-making.
*Status pre-determined by alternative NGS method(s) and compared with SOPHiA DDM™ Dx HRD Solution outcome in up to 233 FFPE ovarian cancer samples processed in 7 sequencing centers on Illumina NextSeq™ 550.
Confidently generate comprehensive reports
SOPHiA DDM™ Dx HRD Solution includes direct access to SOPHiA DDM™ Platform web, a front-end application to upload and analyze genomic sample data and generate downloadable CE-IVD reports. It offers unlimited and unrestricted storage, while keeping patient data safe by applying the highest industrial standards of encryption in compliance with local data security policies.

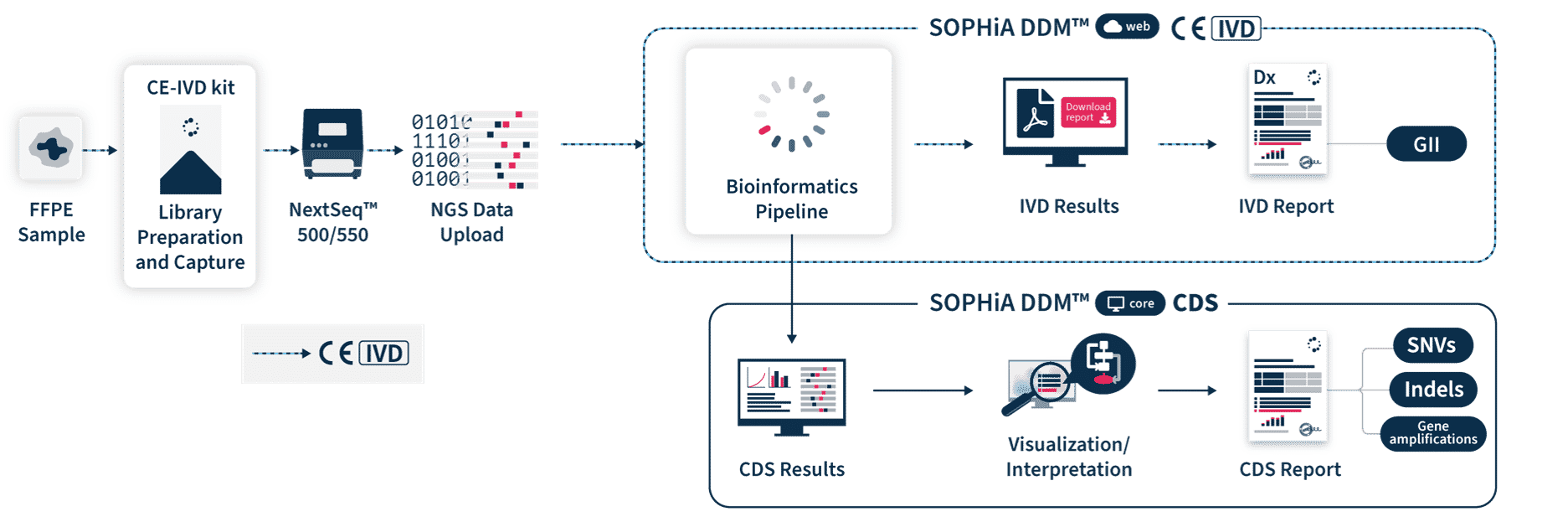
Easily interpret clinically relevant variants
SOPHiA DDM™ Dx HRD Solution offers an additional Clinical Decision Support (CDS*) component via SOPHiA DDM™ Platform core. CDS* features include the detection and visualization of somatic and germline SNVs and Indels as well as gene amplifications in 28 genes associated with HRR, including BRCA1 and BRCA2. Combining the detections of HRR mutations and the GII provides an accurate and sensitive method to determine HRD status of tumor samples. Users also have access to SOPHiA GENETICS™ Community to share valuable knowledge with peers.
Specifications
| Parameters | SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution |
|---|---|
| Diseases Covered | Ovarian cancer |
| Genes (CDS* only) | 28 genes (AKT1*, ATM, BARD1, BRCA1, BRCA2, BRIP1, CCNE1, CDK12, CHEK1, CHEK2, ESR1*, FANCA, FANCD2, FANCL, FGFR1*, FGFR2*, FGFR3*, MRE11, NBN, PALB2, PIK3CA*, PPP2R2A, PTEN, RAD51B, RAD51C, RAD51D, RAD54L, TP53) *Hotspot coverage only. |
| Key Biomarkers | Genomic Integrity Index (GII) |
| Sample Type | FFPE ovarian cancer tissue |
| DNA Input Amount | 50 ng |
| Sequencer Compatibility | Illumina NextSeq™ 500/550 |
| Library Preparation Time | 1.5 days |
| Analysis Time From FASTQ | > 8 hours |
| Detected Variants |
|
| Product type | Molecular diagnostic application (kit + analytics) |
Resources
Fact Sheet and Flyer
Poster
- A multicenter evaluation of a low-pass whole genome sequencing-based solution for homologous recombination deficiency detection
- Clinical relevance evaluation of a novel homologous recombination deficiency CE-IVD decentralized solution that identifies ovarian cancer patients that could potentially benefit from treatment with PARP inhibitors
Contact us
Please fill out the form below to get in touch