SOPHiA DDM™ Dx Hereditary Cancer Solution
Unraveling the genetic ethiology of cancer heredity
Accurate detection and reporting of genetic variants predisposing to cancer are critical for better patient management.
SOPHiA DDM™ Dx Hereditary Cancer Solution (HCS) is a CE-marked in vitro diagnostic (IVD) application based on next-generation sequencing (NGS) enabling accurate and sensitive characterization of the complex mutational landscape associated with major hereditary cancers. Powered by the advanced analytical capabilities of SOPHiA DDM™ Platform, this sample-to-report application helps healthcare professionals increase their efficiency and confidence of getting actionable clinical insights and making data-driven decisions that improve the quality of patient care.
Tailored analytics to accurately detect SNVs and Indels in 27 most common genes involved in predispositions to breast and ovarian cancers as well as colorectal syndromes
Reduced turnaround time with a ready-to-sequence target-enriched library in just 2 days
CE-IVD marked SOPHiA DDM™ Platform web for intuitive reporting with unlimited and safe data storage
Product Details
Accurately detect actionable variants
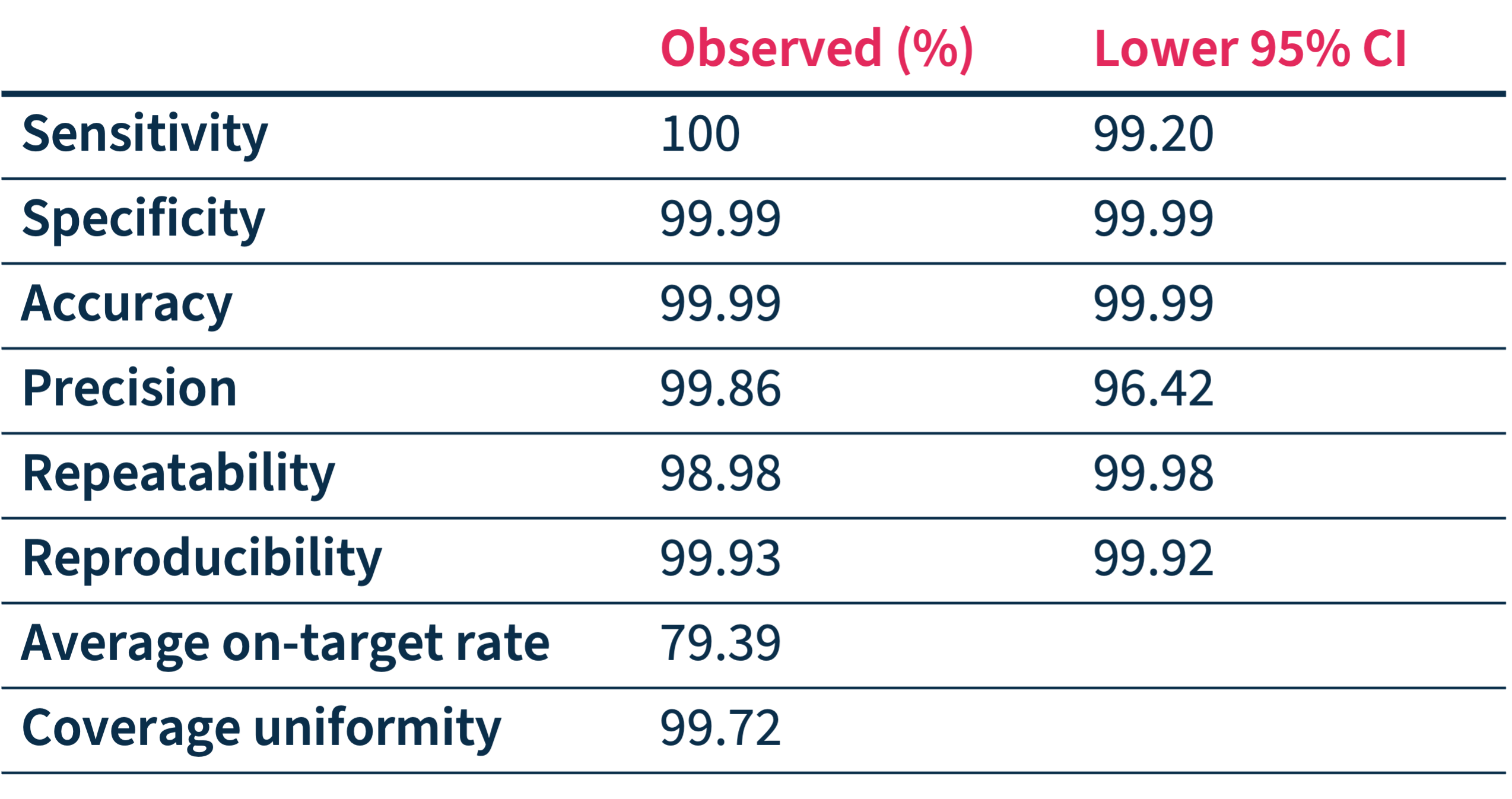
SOPHiA DDM™ Dx HCS accuracy has been assessed through a multicenter performance evaluation study* showing:
- High on-target rates and coverage uniformity
- High-confidence calling of SNVs and Indels in 27 targeted genes
- Identification of PMS2 and PMS2CL variants.
*Performance values have been calculated based on 373 unique variants (SNVs and Indels) in 159 samples processed in 7 sequencing centers on Illumina MiSeq™.

Confidently generate comprehensive reports
SOPHiA DDM™ Dx HCS Solution includes direct access to SOPHiA DDM™ Platform web, a front-end application to upload and analyze genomic sample data and generate downloadable CE-IVD reports. It offers unlimited and unrestricted storage, while keeping patient data safe by applying the highest industrial standards of encryption in compliance with local data security policies.
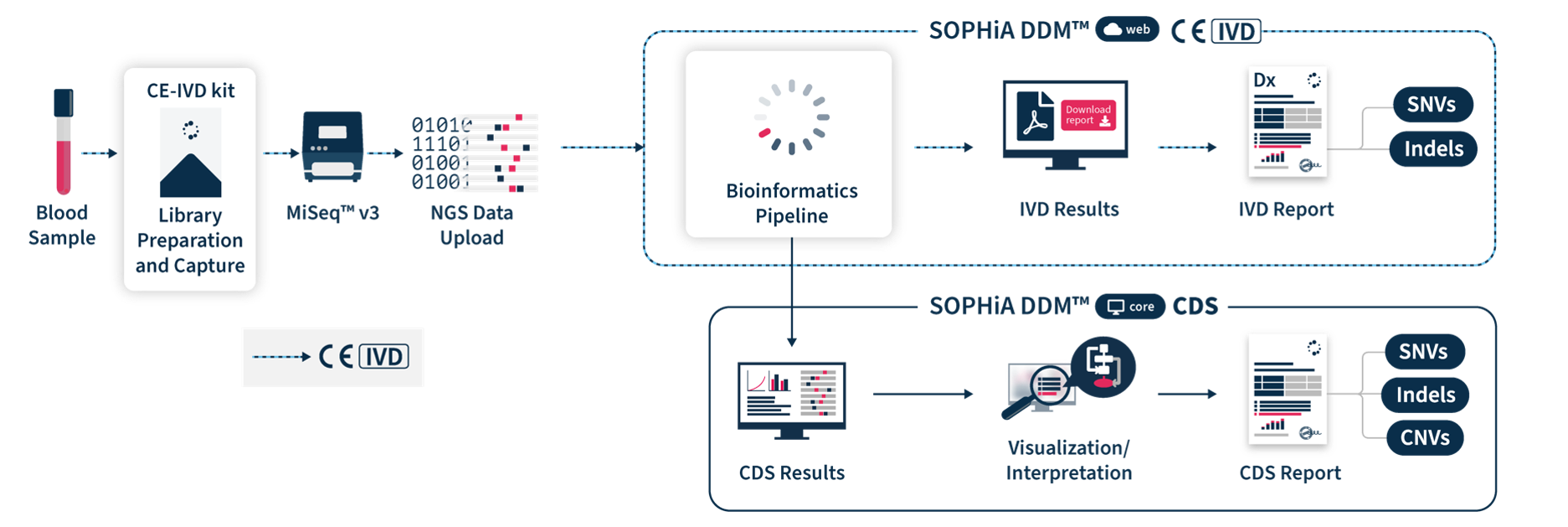
Easily interpret clinically relevant variants
SOPHiA DDM™ Dx HCS offers an additional component via SOPHiA DDM™ Platform core that allows users to visualize, interpret and report key biomarkers. Clinical Decision Support (CDS*) results are computed by the bioinformatics pipeline in a single workflow. CDS* reports include SNVs, Indels and CNVs for the 27 targeted genes and can be tuned via intuitive cascading filters and prioritization options based on databases, in-house machine learning predictions, virtual panels or custom filters. They also feature a comprehensive risk model providing personalised risk estimates via the BRIDGES (Breast Cancer Risk after Diagnostic Gene Sequencing) risk score. Additionaly, users have access to SOPHiA GENETICS™ Community to share valuable knowledge with peers.
Specifications
| Parameters | SOPHiA DDM™ Dx Hereditary Cancer Solution |
|---|---|
| Diseases Covered | Breast and ovarian cancers and colorectal syndromes |
| Genes | 26 genes (ABRAXAS1, ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, MRE11, NBN, PALB2, PIK3CA, RAD50, RAD51C, RAD51D, TP53, XRCC2, EPCAM, MLH1, MSH2, MSH6, PMS2, APC, MUTYH, PTEN, STK11) and PMS2CL pseudogene |
| Target Region SIze | 105 kb |
| Sample Type | Blood |
| DNA Input Amount | 200 ng |
| Sequencer Compatibility | Illumina MiSeq™ |
| Library Preparation Time | 2 days |
| Analysis Time From FASTQ | < 6 hours |
| Detected Variants |
|
| Product type | Molecular diagnostic application (kit + analytics) |
Resources
Contact us
Please fill out the form below to get in touch
Related products
SOPHiA DDM™ Hereditary Cancers Applications
Confidently assess genetic variants predisposing to cancer with our targeted genomic applications.
CE-IVD Oncology Applications by SOPHiA GENETICS™
Transform raw NGS data into actionable insights with our CE-marked in vitro diagnostic (IVD) applications.

CNVs, copy number variants; Indels, insertions and deletions; SNVs, single nucleotide variants.
*CDS – For Clinical decision support use only – not intended as a primary diagnostic tool