Menu
Menu
Push the boundaries of your in-house liquid biopsy research capabilities with MSK-ACCESS® powered with SOPHiA DDM™, a decentralized version of the rigorously validated cell-free DNA (cfDNA) assay developed and used in clinical routine by Memorial Sloan Kettering Cancer Center (MSK)1,2.







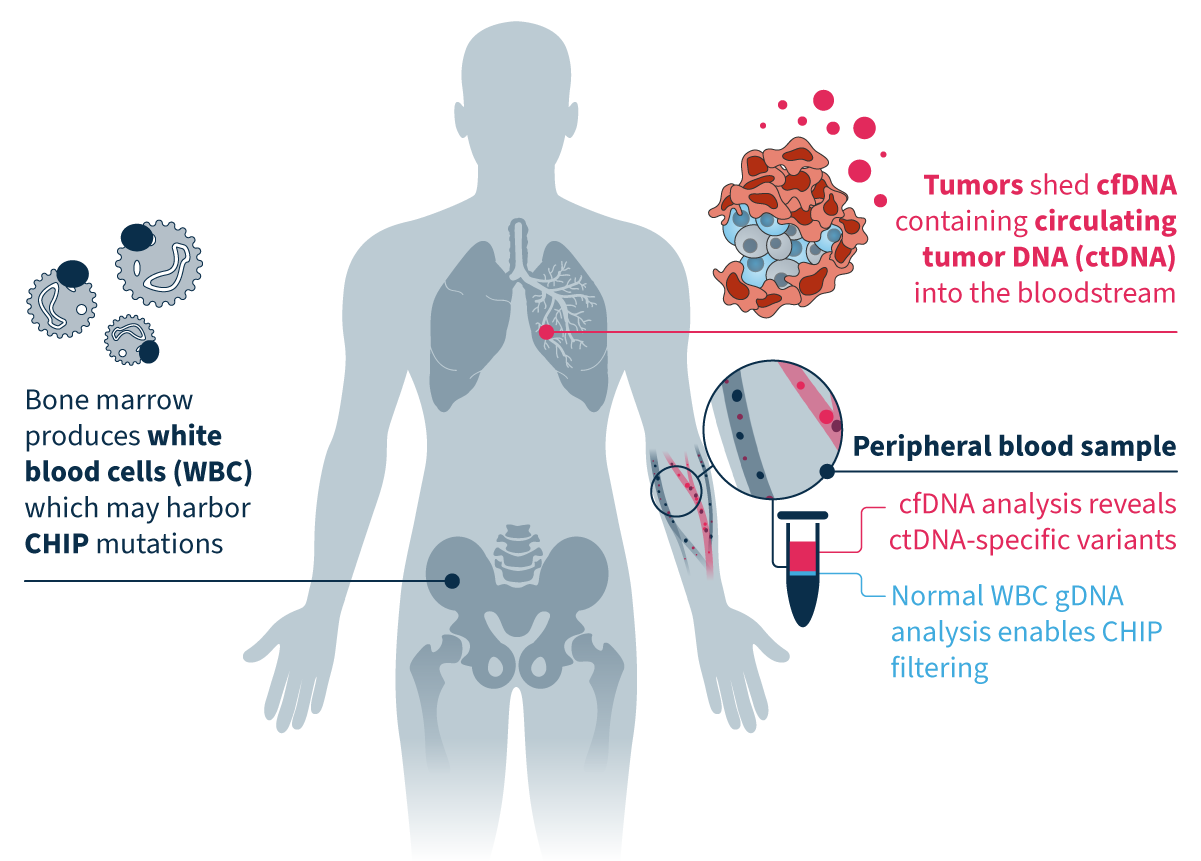
Clonal hematopoiesis of indeterminate potential (CHIP) is a common cause of biological false positives in cfDNA analysis⁴. MSK-ACCESS® powered with SOPHiA DDM™ leverages a matched tumor-normal approach that enables CHIP filtering and eliminates the risk of:
By sequencing tumor-derived cfDNA and normal white blood cell DNA in parallel, you can effectively reduce noise in your data analysis and focus on tumor-specific somatic variants.
Exceptional analytical performance3

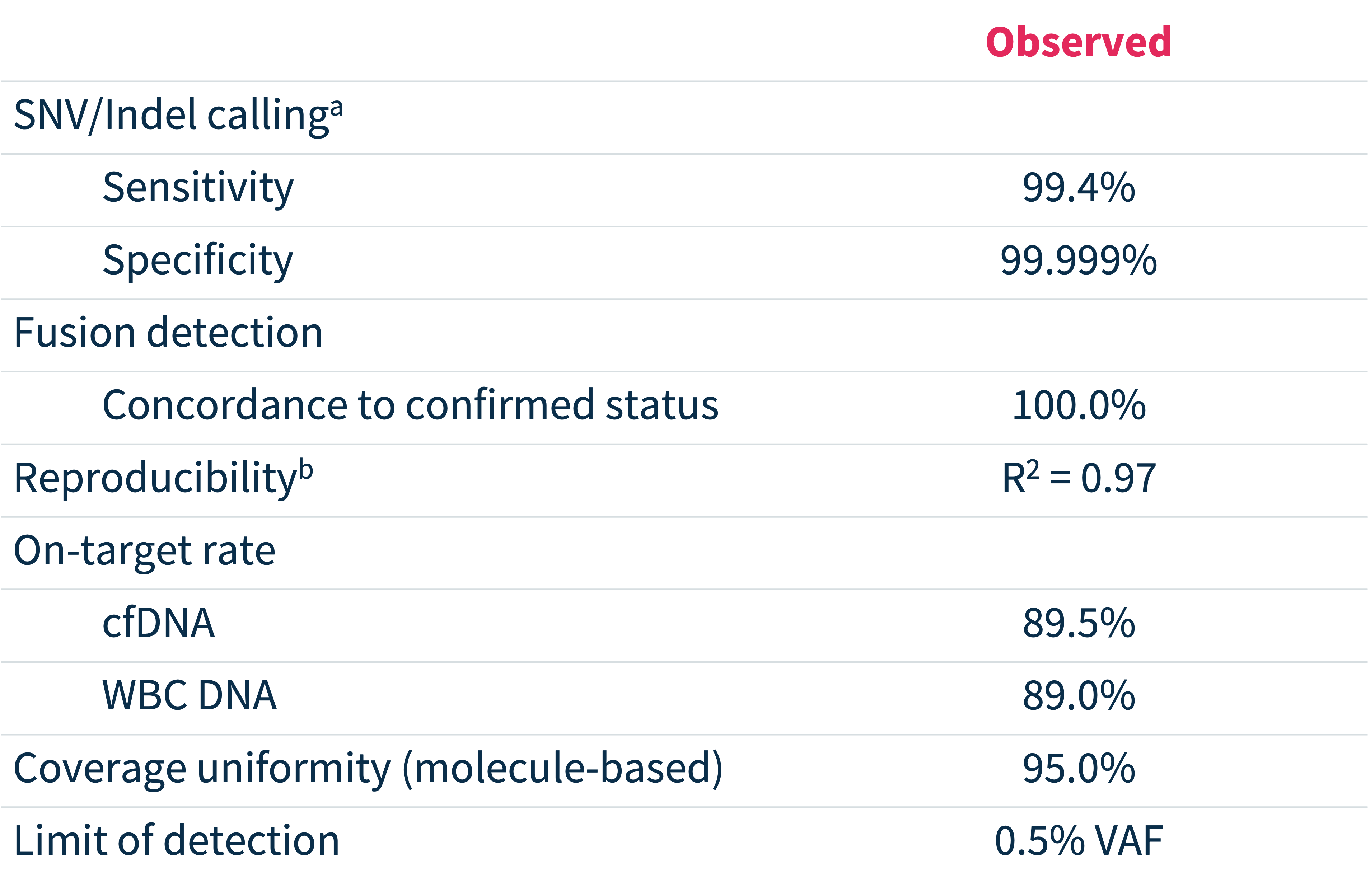
VAF, variant allele frequency. a) Based on end-to-end concordance analysis between MSK-ACCESS® powered with SOPHiA DDM™ and centralized version of MSK-ACCESS® at MSK, using 48 cfDNA + matched WBC DNA samples; b) Correlation of variant allele fraction to reference method.
OncoPortal™ Knowledge Base further supports decision-making and reporting by matching tumor molecular profiles with clinical associations and available clinical trials. In addition, the application offers access to MSK’s Precision Oncology Knowledge Base, OncoKB™, via a link in SOPHiA DDM™ for comprehensive genomic insights.



| Content | 147 genes |
|---|---|
| Diseases Covered | Multi-cancer (any solid tumor) |
| Detected Variants | • SNVs and Indels in 147 genes |
| Sample Type | Paired samples of cell-free DNA extracted from peripheral blood and white blood cell gDNA |
| Starting Material | 20 ng cell-free DNA (minimum); 50 ng white blood cell (WBC) gDNA (minimum) |
| Recommended Read Length | 150 bp, paired end |
| Coverage Depth | Approx. 20,000x |
| Multiplexing (samples per run) | Illumina NovaSeq™ 6000: Illumina NextSeq® 2000 (P4 flow cell): |
| Library Preparation Time | 1.5 days |
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.