Menu
Menu

Liquid biopsy is emerging as a revolutionary technology, offering swift and precise diagnostics. It has the potential to significantly impact precision oncology by providing a non-invasive approach for cancer detection, monitoring, and treatment selection. However, liquid biopsy still faces challenges, including workflow standardization and broad adoption across the globe. Join us for this webinar to […]
Areas of Interest:

Hosted by: SOPHiA GENETICSPresented by: Alexandre HarléProfessor of of Biopathology, Head of Precision Medicine and Translational Research Service, Institut de Cancérologie de Lorraine (ICL), France As precision oncology advances, clinicians and researchers require solid tumor profiling tools that go beyond traditional approaches. While most next-generation sequencing (NGS) solutions rely on DNA as input, RNA sequencing provides unique […]
Areas of Interest:

MSK-ACCESS® powered with SOPHiA DDM™ offers an innovative and decentralized solution that combines MSK’s expertise in cancer genomics with the robust analytics of the SOPHiA DDM™ Platform. This application aims to address the global inequalities in comprehensive cancer care. Watch to hear about the collaborative efforts of SOPHiA GENETICS and AstraZeneca in expanding global access […]
Areas of Interest:

Dr. Hemad Yasaei, Head of Molecular Genomics, National Reference Laboratory (NRL), Abu Dhabi Comprehensive genomic profiling (CGP) of solid tumors has become an essential tool in guiding precision medicine approaches. As cancer research advances, clinical laboratories require fast, scalable, and accurate CGP technologies to detect both known and emerging biomarkers with confidence. Through the decentralized […]
Areas of Interest:

Discover cutting-edge insights with this on-demand session from the ACGH Congress. Dr. Carlos Santamaría, Head of the Molecular Diagnostics Division at the National Children’s Hospital in Costa Rica, shares real-world experiences with exome sequencing in a national pediatric setting. Followed by Dr. Sevana Yaghoubian, Global Director of Genomics at SOPHiA GENETICS, presenting the SOPHiA DDM™ […]
Areas of Interest:

Speaker:Cristovam Scapulatempo Neto, MD, PhD - Medical Director of Pathology and Genetics, Diagnósticos da América S.A. Comprehensive genomic profiling (CGP) is transforming precision oncology by enabling more precise and personalized cancer care. However, as demand for testing grows, many clinical laboratories face significant operational hurdles—from fragmented and rigid analysis workflows to long turnaround times. Decentralized […]
Areas of Interest:

Hereditary cancer risk assessment is a rapidly advancing field, driven by the need for comprehensive variant detection, streamlined genomic workflows, and alignment with evolving guidelines. In the UK, testing is delivered through the NHS Genomic Laboratory Hub network, with each lab supporting a broad range of clinical indications under the National Genomic Test Directory. This […]
Areas of Interest:

As the global healthcare research landscape evolves at lightning speed, we need to change the way we approach healthcare data by breaking down silos, fostering knowledge sharing, and democratizing access to anonymized real-world health insights. And as the volume and complexity of healthcare data continue to grow, the integration of next-generation data analytics and artificial […]
Areas of Interest:

Watch on-demand the webinar “Maximizing Efficiency: Implementing an Enhanced Exome Solution Across Multiple Indications”, where Dr. Eirikur Briem, Head of Department of Genetics and Molecular Medicine at the Landspitali University Hospital in Iceland presents his institute’s experience in implementing the SOPHiA DDM™ Enhanced Exome Solutions.
Areas of Interest:

Filmed as part of the Swiss Biotech Day on May 5th 2025.An engaging panel discussion on the transformative role of AI-powered technology in building scalable, high-performance healthcare solutions. Discover how collaboration drives innovation and advances precision medicine. Discussion summary:Artificial intelligence and big data are revolutionizing biotech and healthcare, driving breakthroughs in precision medicine, clinical decision-making, […]
Areas of Interest:

The diagnostic, prognostic, and treatment landscape of myeloid malignancies is evolving rapidly, with genomic biomarkers increasingly defining disease diagnosis and classification. Given the continuous discovery and refinement of genomic testing requirements in the context of changing (targeted) therapies and reimbursement recommendations, there is an increasing need for genomic solutions that keep pace with clinical and […]
Areas of Interest:

Comprehensive genomic profiling (CGP) using a matched tumor-normal approach can help improve somatic detection rate and streamline interpretation.
Areas of Interest:

This webinar presents an in-depth look at how Memorial Sloan Kettering Cancer Center (MSK) is routinely using its molecular assays — MSK-IMPACT and MSK-ACCESS — together to inform precision oncology approaches.
Areas of Interest:

In this webinar, Silvia Salmoiraghi, biologist at ASST Papa Giovanni XXIII Hospital in Bergamo, Italy, discuss the performance of the SOPHiA DDM™ Residual Acute Myeloid (RAM) Solution.
Areas of Interest:

SOPHiA GENETICS (Nasdaq: SOPH), a cloud-native healthcare technology company and a global leader in data-driven medicine, recently joined the European Liquid Biopsy Society (ELBS), a prestigious network consisting of partners from academia and industry with the common goal of making liquid biopsy tests part of the routine standard of care. SOPHiA GENETICS offers a comprehensive […]
Areas of Interest:

Pinpointing pathogenic mutations from large, complex datasets can be difficult, time-consuming, and somewhat overwhelming. So, how can you streamline your genomic analysis, to make it quicker, easier, and more efficient? In this webinar you will learn how Alamut™ Visual Plus enables clinical researchers to: ➡️ Resolve splice-site variants using splicing scores and exonic splicing enhancer […]
Areas of Interest:

Decoding Complexity – Overcoming Real-World Challenges in Variant Analysis Join us for the second episode of our webinar series, where we delve deeper into the complexities of variant analysis. Our esteemed bioinformatics experts will share practical solutions to real-world challenges in this field. Embark on a journey with us as we explore the advanced strategies […]
Areas of Interest:

Join us for an enlightening webinar on the evolution of pharmacogenetics, from its historical roots to the impact of groundbreaking innovations and the establishment of specialist foundations. We will explore the introduction of crucial guidelines and annotations that have paved the way for the development of key technologies and solutions in this field. Learn how […]
Areas of Interest:

Welcome to the inaugural episode of our new webinar series - Decoding complexity: Tackling real-world challenges in variant analysis. Prepare to embark on an enlightening journey as we tap into the wealth of knowledge possessed by our esteemed bioinformatics experts, who will be sharing practical solutions to real-world challenges in variant analysis. Each installment of […]
Areas of Interest:
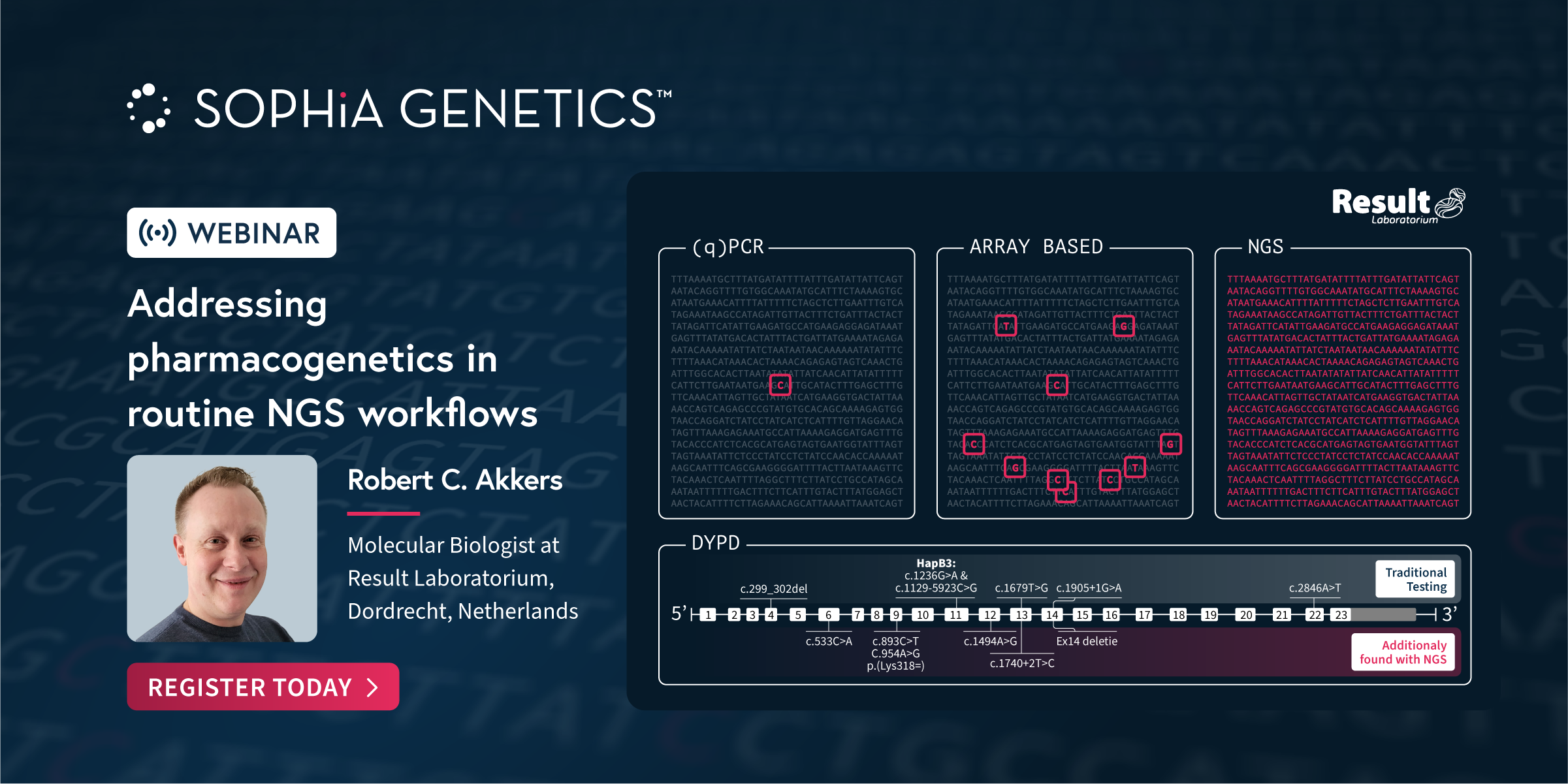
At SOPHiA GENETICS, we collaborate with genetic experts to develop specialized next-generation sequencing (NGS) applications that seamlessly integrate into any laboratory workflow. In this Webinar our partners share how the analytical technology and dedicated features in the SOPHiA DDM™️ Platform have enabled the accurate detection and streamlined assessment of variants associated with Rare Diseases and Pharmacogenomics.Discover how the […]
Areas of Interest:

The webinar aims to provide a comprehensive overview of the latest advancements in the clinical management of high-grade ovarian cancer, with a focus on incorporating information regarding homologous recombination deficiency (HRD) and BRCA statuses obtained through decentralized, in-house assays. Four experts in the field — one anatomic pathologist, two medical oncologists, and one biologist — will present selected […]
Areas of Interest:
Going beyond HRR mutations: A deep-learning approach on HRD detection in ovarian cancer Homologous recombination deficiency (HRD) is an important prognostic and predictive biomarker in ovarian cancer. It is assessed by combining information from homologous recombination repair (HRR) gene mutations, the “cause” of HRD, with a measure of genomic scarring, the “effect” of HRD. However, […]
Areas of Interest:

SOPHiA GENETICS is collaborating with Memorial Sloan Kettering Cancer Center (MSK) to decentralize their advanced precision oncology tools – MSK-ACCESS® for liquid biopsy and MSK-IMPACT® for comprehensive genomic profiling (CGP). By combining the clinical expertise of MSK in cancer genomics, the predictive algorithms of SOPHiA DDMTM, and the power of the global SOPHiA GENETICS network, […]
Areas of Interest:

Optimized variant prioritization for enhanced insights: SOPHiA DDM™️ and Alamut™️ Visual Plus in Action.Are you keen to improve your tertiary analysis? Discover how SOPHiA GENETICS end-to-end workflows can do just this.Our webinar covers: Presented by:
Areas of Interest:
The critical role of transcript analysis for refining the classification of variants associated with constitutional disorders. Alamut™️ Visual Plus user presentation at the ASHG 2023 Annual Meeting. Presented by: Kai Lee Yap, PhD, FACMG,Director of Molecular Diagnostics, Ann & Robert H. Lurie Children’s Hospital of Chicago, Assistant Professor of Pathology, Northwestern University Feinberg School of Medicine.
Areas of Interest:

Lymphoid neoplasms encompassing lymphomas and some leukemia like Chronic Lymphocytic Leukaemia (CLL) are the most common type of blood cancer . With increasing evidence for the stratification of tumor types with distinct clinical and biological features according to biomarkers, and the progress in targeted therapy, tailored NGS-based workflows empower experts to get high-quality and reproducible […]
Areas of Interest:

Areas of Interest:

Areas of Interest:

What’s New? CE-IVD Oncology Applications by SOPHiA GENETICS™ Alexander Kurze, PhD Senior Director Product Management, SOPHiA GENETICS Evaluation of a low-pass whole genome sequencing-based solution for homologous recombination deficiency detection supported by deep learning algorithms Dr. Adrien Buisson Praticien Spécialiste des CLCC – UF de Biologie des Tumeurs, Centre Léon Bérard- Cheney
Areas of Interest:

About Gene fusions are the latest type of biomarker to receive broad applicability in cancer management. More than 10,000 gene fusions have already been identified in human cancers and it is estimated that up to 80% of solid tumors could benefit from gene fusion testing. The number of new drug approvals in fusion-positive cancers has […]
Areas of Interest:

About Deficiency in the homologous recombination repair system represent up to 50% of the ovarian, breast, prostate and pancreatic cancers. While Poly ADP-ribose polymerase inhibitors (PARPi) treatment revolutionized management of patients, inducing synthetic lethality in cells with homologous recombination deficiency (HRD), detection of HRD is challenging. Indeed, homologous recombination repair (HRR) genetic testing do not […]
Areas of Interest:

About Gene fusions are the latest type of biomarker to receive broad applicability in cancer management. More than 10,000 gene fusions have already been identified in human cancers and it is estimated that up to 80% of solid tumors could benefit from gene fusion testing. The number of new drug approvals in fusion-positive cancers has […]
Areas of Interest:

Nosso webinar tem como foco apresentar os avanços na caracterização do perfil molecular de tumores. Contaremos com palestras e discussões envolvendo nossos gerentes de produtos e especialistas no assunto, abordando os seguintes tópicos: A missão da SOPHiA em democratizar a medicina baseada em dados Uma nova abordagem para detecção rápida e precisa de fusões em […]
Areas of Interest:

En el 2018 una red de colaboradores y especialistas en cáncer en América Latina unió esfuerzos para crear el Consorcio Latinoamericano para el Estudio del Cáncer de Mama y Ovario hereditario (LACAM). El Consorcio LACAM tiene como objetivo reclutar 3,000 casos de múltiples centros en: Argentina, Colombia, Guatemala, México, Paraguay y Perú para desarrollar el […]
Areas of Interest:

Areas of Interest:

Areas of Interest:
Areas of Interest:
Areas of Interest:

Areas of Interest:

Areas of Interest:
This webinar will discuss how Moffitt Cancer Center has implemented a new capture-based application to accurately assess myeloid malignancies by detecting complex variants in challenging genes in a single experiment. Molecular profiling by next-generation sequencing (NGS) of myeloid tumors has become a routine part of disease management. One of the difficulties and limitations of NGS […]
Areas of Interest:

Areas of Interest:

Areas of Interest:

Areas of Interest:

Areas of Interest:

This talk was presented at CGC 2021 on August 2nd, 2021. Join our experts to learn more about how the SOPHiA DDM™ platform supports solid tumor detection.
Areas of Interest:

Areas of Interest:

Areas of Interest:
SOPHiA GENETICS products are for Research Use Only and not for use in diagnostic procedures unless specified otherwise.
SOPHiA DDM™ Dx Hereditary Cancer Solution, SOPHiA DDM™ Dx RNAtarget Oncology Solution and SOPHiA DDM™ Dx Homologous Recombination Deficiency Solution are available as CE-IVD products for In Vitro Diagnostic Use in the European Economic Area (EEA), the United Kingdom and Switzerland. SOPHiA DDM™ Dx Myeloid Solution and SOPHiA DDM™ Dx Solid Tumor Solution are available as CE-IVD products for In Vitro Diagnostic Use in the EEA, the United Kingdom, Switzerland, and Israel. Information about products that may or may not be available in different countries and if applicable, may or may not have received approval or market clearance by a governmental regulatory body for different indications for use. Please contact us to obtain the appropriate product information for your country of residence.
All third-party trademarks listed by SOPHiA GENETICS remain the property of their respective owners. Unless specifically identified as such, SOPHiA GENETICS’ use of third-party trademarks does not indicate any relationship, sponsorship, or endorsement between SOPHiA GENETICS and the owners of these trademarks. Any references by SOPHiA GENETICS to third-party trademarks is to identify the corresponding third-party goods and/or services and shall be considered nominative fair use under the trademark law.